United Therapeutics UTHR announced that the FDA has issued a complete response letter to its new drug application (“NDA”) for its drug device combination therapy, Tyvaso Dry Powder Inhaler or Tyvaso DPI.
– Zacks
The NDA is seeking approval for the candidate to treat pulmonary arterial hypertension (“PAH”) and pulmonary hypertension associated with interstitial lung disease (“PH-ILD”). Tyvaso DPI comprises the dry powder formulation of United Therapeutics’ PAH medicine, Tyvaso (treprostinil), and a small, portable, dry powder inhaler.
Per the complete response letter (“CRL”), the FDA identified a deficiency related to an open inspection issue at a third-party facility that performs analytical testing of treprostinil drug substance. This prevented the FDA from approving the NDA in its present form.
United Therapeutics has developed Tyvaso DPI in collaboration with MannKind Corporation MNKD. The FDA did not report any deficiencies in its CRL with regard to operations at MannKind’s device manufacturing and testing facility for Tyvaso DPI.
Shares of both Mannkind and United Therapeutics were down 18.3% and 1.5%, respectively, in intra-day trading on Oct 18, following the above news. Nonetheless, in the year so far, United Therapeutics’ shares have rallied 21.4% against the industry’s 16.9% decline.
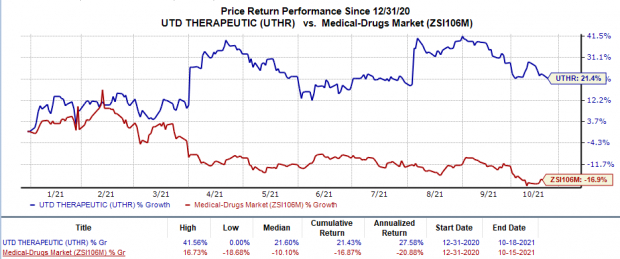 Image Source: Zacks Investment Research
Image Source: Zacks Investment Research
Following the CRL, the company anticipates that the FDA’s query regarding the deficiency can be resolved quickly and it can obtain approval for Tyvaso DPI in the United States by the summer of 2022.
The drug-device product offers some benefits over nebulized Tyvaso Inhalation Solution, which is already approved in the United States for the treatment of PAH and PH-ILD. Tyvaso DPI is expected to be less time-consuming to administer, and more mobile and convenient. The NDA filing was based on data from the BREEZE study.
If approved, United Therapeutics expects Tyvaso DPI to double the number of patients on Tyvaso by 2022.
Please note that United Therapeutics filed the NDA for Tyvaso DPI earlier this year in April with a priority review voucher. The FDA accepted the NDA and granted priority review to the NDA in June 2021.
Please also note that United Therapeutics is a market leader in PAH medicines. Other than Tyvaso, which is an inhaled version of treprostinil, the company markets three other PAH medicines in the United States — Remodulin, an injectable formulation of treprostinil approved for both subcutaneous and intravenous use; Orenitram, an oral version of treprostinil; and Adcirca tablets. United Therapeutics bought exclusive rights to commercialize Adcirca (tadalafil) for PAH in the United States from Eli Lilly LLY in November 2008. Eli Lilly markets tadalafil as Cialis for erectile dysfunction. Adcirca/Cialis lost exclusivity in 2018 and generic versions are available.
Zacks Rank & Stock to Consider
United Therapeutics currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the same sector is Xencor XNCR, which carries a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Xencor’s earnings per share estimates for 2021 have narrowed from $0.75 to $0.33 in the past 60 days. The same for 2022 has narrowed from $3.14 to $2.92 over the same period.
(We are reissuing this article to correct a mistake. The original article, issued on October 19, 2021, should no longer be relied upon.)
Zacks’ Top Picks to Cash in on Artificial Intelligence
This world-changing technology is projected to generate $100s of billions by 2025. From self-driving cars to consumer data analysis, people are relying on machines more than we ever have before. Now is the time to capitalize on the 4th Industrial Revolution. Zacks’ urgent special report reveals 6 AI picks investors need to know about today.
See 6 Artificial Intelligence Stocks With Extreme Upside Potential>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
MannKind Corporation (MNKD): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
United Therapeutics Corporation (UTHR): Free Stock Analysis Report
Xencor, Inc. (XNCR): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research



